easiest reaction ever!
I'll admit I don't have much to do with green chemistry. Sure, whatever, I'll use it if it'll do what I want done, but I'm not actively involved in green research. I've become a fan of it lately, though.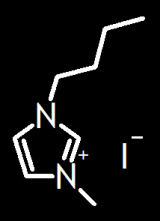 So this is the first ionic liquid I've ever had occasion to use. I also had to make it. (First time I've ever had to synthesize a solvent. Guess there's a first time for everything, eh?) Didn't mind, though, because the starting materials were already on the shelf. These guys over at Los Alamos & Oak Ridge made
So this is the first ionic liquid I've ever had occasion to use. I also had to make it. (First time I've ever had to synthesize a solvent. Guess there's a first time for everything, eh?) Didn't mind, though, because the starting materials were already on the shelf. These guys over at Los Alamos & Oak Ridge made
1-Butyl-3-methylimidazolium bromide, which was pretty close to what I wanted. They used one of the reagents as solvent, which is a pretty cool idea and which also means you get to avoid the rotavap.[1] They claim the addition is exothermic, which I guess is a concern on a larger scale (which they had to deal with and I didn't). Add the iodobutane kinda slowly and then let it stir for a day or so.[2]
So it's stupid-easy to run, which makes me happy. They list a purification method that involves charcoal and a lyophilizer, but I didn't have one of those handy. For my purposes, though, trace impurities are not that much of a concern.[3] The NMR was the cleanest I've seen in a while.
Hopefully it will actually work in the reaction I'm going to throw it into.
[1] Don't know about you, but I hate waiting for solvent to evaporate. I'm not going to live forever, you know. I don't want to waste very much of that time watching a roundbottom spin, but invariably I do...
[2] For paranoia reasons, I had this one under nitrogen...another reference mentioned a problem with the iodide getting oxidized or something.
[3] The stuff they made was really super extra squeaky clean.

12 comments:
I made the chloride version, it is a liquid also. I used an excess of butyl chloride (it is quite volatile) and a pressure flask, it needed to be heated.
NMR can easily miss 2% of an impurity.
My favorite ionic liquid is Me3N.HCl.(AlCl3)x where x=from 1 to 2. You mix two solids and they melt with a tremendous heat evolution. A great reagent, to cleave those pesky methyl aryl ethers, to deprotect phenol OH groups.
Aww, bad link is bad:
"purchase article," it says. :(
I can live with 2% or even a little more. After all, TCI lists ~97% purity at $55 for 5g. Both before and after the step I need the quick & dirty ionic liquid for, I get gooey inseparable mixtures of crud! Yay! (Fortunately, it can all be removed at the end.)
"gooey inseparable mixtures of crud"
as opposed to ionic liquids?
Hmm, good point I guess...it certainly is thick enough.
what is the starting material? methylimidazole? btw, really nice informative post! stupid-easy to skip.
Cool! I always DREAM to do an IL-based project. Now I'm looking for IL-doing professors all over the world.
"Don't know about you, but I hate waiting for solvent to evaporate"
And I hate waiting in the grocery store line :(
And I hate waiting in general...
We bought pressure regulators for the pump, they cost around $900 and work great to prevent bumping. Except that the cut-off valve in my unit just got eaten by evaporating HCl this Tuesday - but I found out that just adding a needle valve inbetween my busted regulator and the pump, I can do a pretty good job at manual control and after adjusting the valve I still don't have to stand there and watch my rotavap spinning.
I used to make quite a few substituted imidazoles, especially N,N'-alkylated ones.
The first alkylation is always troublesome and gives pathetic yields. I prefer to form the ring using the method in Arduengo's patent (glyoxal, ammonium acetate, paraformaldehyde, and R-NH2), ~ 30 % or so after recrys, but with no side products.
Lab lore is that the second alkylation is convenient in refluxing toluene — the product is easily precipitated. The alternative was to use neat reactants (i.e. no solvents). In my hands, the trick was acetonitrile, reflux for 2-3 days.
Hope this helps?
It is worth being a little careful with the imidazoles. While applying for TSCA registration for the BMI Chloride, we received a note from EPA stating that the chloride salt was highly toxic to rats through the skin.
The neat prep of the Cl salt works very well, though as you get close to completion the viscosity increases so much that the rate of rxn slows way down.
Post a Comment