I Can See Clar-ly Now, Part 2: Hexacene's Revenge
Not too long after my lil' unsolicited advice post on Clar's aromatic sextets comes this Org. Lett. paper in ASAP today about the synthesis of hexacene. (While Ψ*Ψ has much more experience with linear acenes, I think she's out of town, so I'm scooping her on this one. Sorry hon.)
Hexacene is a green polycyclic aromatic hydrocarbon (PAH) with six fused, six-membered rings. In PAH's, the number and position of aromatic sextets (that is, benzene-like 6π electron systems) determines the reactivity of the PAH. As is the case with all linear acenes, such as naphthalene, anthracene, and pentacene, hexacene has only one aromatic sextet dispersed about the whole molecule. In other words, it's VERY reactive. It reacts with molecular oxygen readily, and will also rapidly dimerize to form a butterfly-looking molecule. It will even react with bananas.*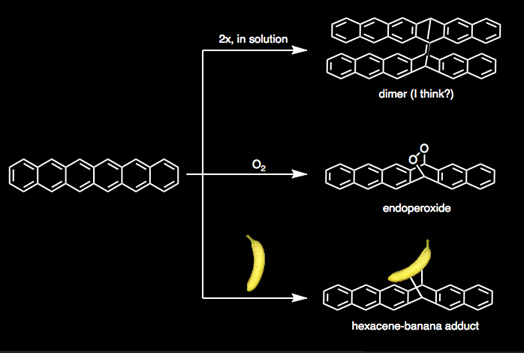
(Ψ*Ψ may want to school me on what the dimer actually looks like.)
You might be wondering how one even goes about these long, linear acenes. Well, hexacene is so reactive that the amount of information in the literature on it is scarce, and previous syntheses of it are inconsistent at best. Functionalized hexacenes of marginal stability have been synthesized and characterized, however. Nonetheless, these long linear acenes are of particular interest because of their interesting electronic properties and potential applications in organic field-effect transistors (oFETs).
The Shah and Neckers groups at BGSU have found a nicer alternative to previous syntheses of hexacene, and even found a cute way to keep it around long enough to characterize it. Using a reaction known as the Strating−Zwanenberg photodecarbonylation as the final hexacene-making step, they were able to make hexacene in a paltry six steps. Hexacene only stuck around for a couple minutes in solution before deciding to dimerize or react with oxygen. It was long enough, however, to get a nice UV/Vis and mass spec on it. It's green! Whee!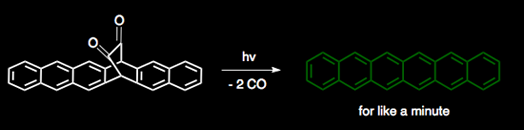
A consistent synthesis of hexacene alone was probably enough to get this paper in Org. Lett., but then they went a step further. Incorporating the dione into a solid matrix of poly(methyl methacrylate) and irradiation formed hexacene. The polymer matrix kept the hexacene from dimerizing or reacting with oxygen for about 12 hours, until oxygen started to creep in.
So there you have it! Isn't aromatic chemistry FUN? Just remember one of Clar's findings if you deal with them: the prettier the PAH, the less likely it'll be around for long.
*Yes, this shit IS bananas. B-A-N-A-N-A-S.

10 comments:
What? Something new on acenes and I haven't seen it? Oh noes!
...I've been in the habit of not posting much of anything directly related to the main focus of my group's research, but since I clearly no longer care (see also: sidebar?), that may change.
PAHs sound suspiciously like girls in my dorm room back in my college days...
Do you mean the "dimerizing" part or the only sticking around for like a minute part?
I thought he meant the "reacts with anything" part...
hexacene only sticks around for a minute or two before dimerizing or reacting with oxygen.
I was going with the "the prettier the PAH, the less likely it will stick around" part.
You could substitute "drunken co-ed" for "PAH", if you'd like.
yep, -2CO is one of the things that happened with my stuff that I described here
I'm sad that noone has mentioned the bananadduct. *sigh* I still giggle at that.
Come on, it's just too well-known that long linear acenes react with bananas!
Hi, an interesting paper on high acene stabilization has been published on J.Mater.Chem., 2007,17,2636
Post a Comment