oxygen--part one
I mentioned singlet oxygen a while back in the context of solar cell materials. However, I didn't get to go into specifics, and unlike solid-state chemistry (which is too close to solid-state physics for my tastes), photochemistry is among my current obsessions. While I can't quite put this in completely plain non-chemist English, I think I can make it manageable for chemists who can't do quantum.

You know, the one where you pour liquid oxygen over the poles of a really strong magnet...and it sticks? Looks like a little donut? (By contrast, if you do the same thing with liquid nitrogen, nothing this exciting happens.)
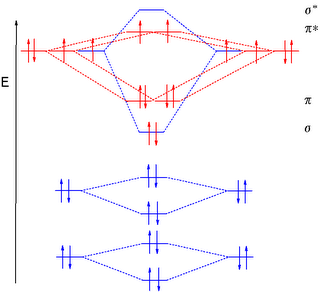
Back to the orbital diagram. Look at the pi-antibonding electrons. Imagine "flipping" one of them so that the spins points the other way. This is the ¹Σg+ state. While it is a singlet state (note the "1" multiplicity instead of the "3" for the ground state), it's actually the second excited state. This one actually doesn't get much consideration, because it decays so quickly to the first excited state that there isn't time enough for it to do anything interesting.
So what are the electrons doing in the first excited state? Back to the pi-antibonding electrons in the diagram. Now visualize putting both of them into the same orbital and making the spins antiparallel. This is ¹Δg oxygen. It is what's commonly referred to as singlet oxygen.
Why does this matter? Think of most organic compounds in their ground state. Absorbing a little light in the UV or visible range will immediately kick them up into an excited state. Of course, most organic compounds (radicals and triplet weirdos excluded!) are ground-state singlets, so their electrons have paired spins in the first place. And the excited state they reach when you add light is also a singlet. This is not accidental. It's also something oxygen can't do. This is because there are NO SPIN FLIPS DURING EXCITATION! As a consequence, triplet oxygen cannot be directly excited to the singlet state. (This is good news for us, since reactive oxygen species are not beneficial to our continued living.)
Next: how is singlet oxygen produced?

1 comment:
Nice MO diagram - definitely worth tattoing somewhere ;)
Post a Comment