Self-cleaning cotton ftw
As Ψ*Ψ showed in the last post, pretty colors are awesome. But sometimes, pretty colors are better off in the flask than, say, on your new white shirt. And ugly colors are frankly never appreciated anywhere. Imagine the following situation: you're in lab, drinking coffee and working, it's midnight, and you spill some coffee on your lucky synthesis shirt- a white shirt you got for free from some hot female (or male) sales rep at PittCon. Well crap, now if you want to clean it, you have to go home and do a load of laundry and you're running a column right now and... well, looks like your lucky shirt is now your lucky coffee-stained shirt. "What if," you think... "What if I never had to clean this shirt again?"
Nanocrap to the rescue! A textile group in Hong Kong has developed a type of fabric they call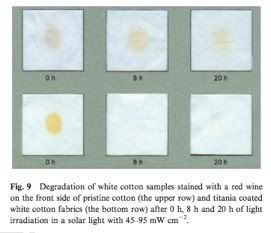 self-cleaning cotton. In it, cotton is doped with nanocrystalline TiO2 that act as self-cleaning agents. Upon irradiation with sunlight for a few hours, the titania acts as a photobleaching agent to remove common stains such as red wine and coffee. Furthermore, the titania possessed antibacterial properties, rendering the fabric truly self-cleaning, from both a colorimetric and microbial perspective. However, the casting process to embed these titania crystals in the cotton weakens the structure of the cotton, rendering the cotton more prone to tearing. Furthermore, I'm not sure how titania would react to your skin, so wearable self-cleaning cotton adds a whole new challenge to the mix. Still, this is a pretty awesome start to a interesting field, and one that blends nanocrap with materials with useful properties.
self-cleaning cotton. In it, cotton is doped with nanocrystalline TiO2 that act as self-cleaning agents. Upon irradiation with sunlight for a few hours, the titania acts as a photobleaching agent to remove common stains such as red wine and coffee. Furthermore, the titania possessed antibacterial properties, rendering the fabric truly self-cleaning, from both a colorimetric and microbial perspective. However, the casting process to embed these titania crystals in the cotton weakens the structure of the cotton, rendering the cotton more prone to tearing. Furthermore, I'm not sure how titania would react to your skin, so wearable self-cleaning cotton adds a whole new challenge to the mix. Still, this is a pretty awesome start to a interesting field, and one that blends nanocrap with materials with useful properties.
Just think: in a few years you'll have one less reason to go home. This is either a good or a bad thing, depending on your boss' thoughts on the matter.

6 comments:
Hmmmmm. Considering titania is, like, THE white pigment...I wouldn't be too surprised if it were in some cosmetics. Just speculation.
Yep, it is.
It's in tooth-cremes, sunblockers, etc. You could find it almost everywhere.
While titania might not be that nontoxic, I wonder what the effects of extremely small particles of titania would be. I would imagine they could penetrate and irritate skin, like silica gel can. Might not be toxic per se, but there are other considerations.
er, not that toxic.
It's a nice idea on the side of the application but not totally new in the concept. I think I have heard/read something about the use of photochemistry in self-cleaning agents. One was to cover ceramic tiles to be applied in walls (as in the bathroom or kitchen) with a film incorporating these agents to eliminate "grease" and smell. The other was to make a film on the glass windows of cars (I think this one is already being used, if I'm not mistaken, by Honda ).
Do you know something about it?
Post a Comment