Organic solar cells in plain english--part one
Solar cells of the cheap plastic variety (carbon rather than silicon) are "up there" in my long, long list of side interests. There are some obvious problems, though. While this is nice for those of us who like to research them (can't study something if it's already perfect), it's not so nice for commercial applications. Solar cells that are flexible or that can be sprayed on are cool as hell, but no one will buy them (save for hardcore gadget nuts) if they only last a few days or so. In the next few posts, I'll attempt to explain in straight english how these things fall apart. Shockingly low efficiency is another issue for another post as well, maybe. First things first. How does a solar cell work? Correct me if I'm wrong. Please? Next time: more about the active layer, and the eeeeevils of oxygen.
First, let me clarify that I'm only talking about a specific kind of solar cell. Most people are familiar with the silicon-based cells in their calculators and elsewhere. There are also hybrids and dye-sensitized (Gratzel) solar cells, which are cool, but my carbon fixation leads me to focus on these, the all-organic kind.
Here's a picture of a typical device. ITO/glass is pretty standard and is used by most people. ITO stands for indium tin oxide. Being both transparent and conductive makes it attractive for use as an electrode in a solar cell. PEDOT:PSS is a conductive polymer used as a hole transport layer. The active layer contains a donor (p-type material) and an acceptor (n-type material). The acceptor is usually C60 or PCBM (derived from C60). The donor gets a lot of attention, and there are a number of materials that have been developed to serve this purpose. BCP serves as an electron transport layer, but other materials can be used instead. Aluminum is the other electrode. Silver or calcium can substitute here.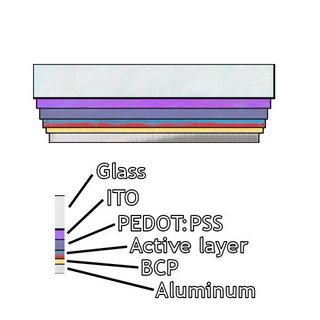 So how do you get energy out of these? The active layer is where the photoinduced charge transfer takes place. Photoinduced charge transfer involves light--a photon enters the active layer and creates an exciton (electron-hole) pair. The electron diffuses to one electrode and the hole to the other. There you go--a voltage difference between the electrodes. For those of us who don't remember physics, the voltage difference is crucial if you want to be able to use your solar cell to power anything. What do you call a battery with no voltage difference across the terminals? Dead.
So how do you get energy out of these? The active layer is where the photoinduced charge transfer takes place. Photoinduced charge transfer involves light--a photon enters the active layer and creates an exciton (electron-hole) pair. The electron diffuses to one electrode and the hole to the other. There you go--a voltage difference between the electrodes. For those of us who don't remember physics, the voltage difference is crucial if you want to be able to use your solar cell to power anything. What do you call a battery with no voltage difference across the terminals? Dead.

No comments:
Post a Comment